Water, a substance composed of the chemical elements hydrogen and oxygen and existing in gaseous, liquid, and solid states. It is one of the most plentiful and essential of compounds. A tasteless and odourless liquid at room temperature, it has the important ability to dissolve many other substances. Indeed, the versatility of water as a solvent is essential to living organisms. Life is believed to have originated in the aqueous solutions of the world’s oceans, and living organisms depend on aqueous solutions, such as blood and digestive juices, for biological processes. In small quantities water appears colourless, but water actually has an intrinsic blue colour caused by slight absorption of light at red wavelengths.

Water is the most plentiful compound on Earth and is essential to life. Although water molecules are simple in structure (H2O), the physical and chemical properties of water are extraordinarily complicated.
Although the molecules of water are simple in structure (H2O), the physical and chemical properties of the compound are extraordinarily complicated, and they are not typical of most substances found on Earth. For example, although the sight of ice cubes floating in a glass of ice water is commonplace, such behaviour is unusual for chemical entities. For almost every other compound, the solid state is denser than the liquid state; thus, the solid would sink to the bottom of the liquid. The fact that ice floats on water is exceedingly important in the natural world, because the ice that forms on ponds and lakes in cold areas of the world acts as an insulating barrier that protects the aquatic life below. If ice were denser than liquid water, ice forming on a pond would sink, thereby exposing more water to the cold temperature. Thus, the pond would eventually freeze throughout, killing all the life-forms present.
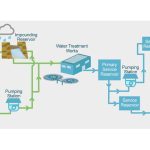
Water occurs as a liquid on the surface of Earth under normal conditions, which makes it invaluable for transportation, for recreation, and as a habitat for a myriad of plants and animals. The fact that water is readily changed to a vapour (gas) allows it to be transported through the atmosphere from the oceans to inland areas where it condenses and, as rain, nourishes plant and animal life. (See hydrosphere: The hydrologic cycle for a description of the cycle by which water is transferred over Earth.)

In the hydrologic cycle, water is transferred between the land surface, the ocean, and the atmosphere. The numbers on the arrows indicate relative water fluxes.
Because of its prominence, water has long played an important religious and philosophical role in human history. In the 6th century BCE, Thales of Miletus, sometimes credited for initiating Greek philosophy, regarded water as the sole fundamental building block of matter:
Two hundred years later, Aristotle considered water to be one of four fundamental elements, in addition to earth, air, and fire. The belief that water was a fundamental substance persisted for more than 2,000 years until experiments in the second half of the 18th century showed that water is a compound made up of the elements hydrogen and oxygen.
The water on the surface of Earth is found mainly in its oceans (97.25 percent) and polar ice caps and glaciers (2.05 percent), with the balance in freshwater lakes, rivers, and groundwater. As Earth’s population grows and the demand for fresh water increases, water purification and recycling become increasingly important. Interestingly, the purity requirements of water for industrial use often exceed those for human consumption. For example, the water used in high-pressure boilers must be at least 99.999998 percent pure. Because seawater contains large quantities of dissolved salts, it must be desalinated for most uses, including human consumption.